We use cookies to enhance your browsing experience, serve personalized ads or content, and analyze our traffic. By clicking "Accept All", you consent to our use of cookies.

Over the last few years, data has quickly become one of the most effective drivers of innovation and growth for organizations operating within the sphere of Healthcare and Life Sciences, and a significant amount of Real-World Data (RWD) is actively being generated outside of clinical trials. The ability to harness, house and interpret this RWD gives institutions an indispensable advantage in these increasingly competitive industries.
Real-World Evidence (RWE) can be derived from sets of RWD such as Electronic Health Records (EHRs), patient reported data, claims and billing information, and disease and product registries. The FDA describes RWE as clinical evidence regarding the usage and potential benefits or risks of a medical product derived from the analysis of RWD.
When properly extrapolated, these insights can play a crucial role in treatment selection within essential healthcare delivery settings. Additionally, this RWE can also have a notable impact on the approvals process as well as the acceleration of drug development. This all leads to higher standards of care and improved health outcomes for individual patients, while simultaneously lowering costs for the institutions developing these treatments.
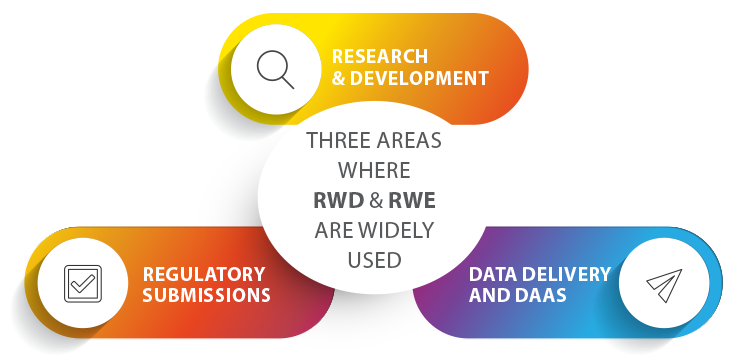
Generally speaking, these opportunities can be realized in three major areas: the first of which being Research and Development, where data such as genetic information can help identify the best therapeutic targets. Real-World Data can help inform research, design and decisions with vital information regarding the connection between phenotypic expression of diseases and clinical decision-making. RWD can also help steer research in the right direction during the early stages of drug development by narrowing a large field of potential targets down to just a few high quality candidates, saving both time and money.
The second of these opportunities is in regulatory submissions, the series of documents and data sent by drug companies to a health authority as evidence of compliance. Laws and regulations influence many aspects of the drug development process by impacting how drug companies manufacture their drugs, design clinical trials, and report safety findings. Over the last five years, the FDA has created and expanded criteria for using Real-World Data in the approval process. This data can be combined with data from clinical trials in new FDA submissions and as support for new indications of therapies that have already been approved. The supplementation of RWD vastly accelerates the approval process and reduces the overall cost of clinical trials. This is especially crucial when it comes to getting treatments for rare diseases to market, since those clinical trials are faced with the challenge of having much smaller patient pools to recruit from.
The final major opportunity is in tailored data delivery and DaaS, or Data as a Service. This generally encompasses a variety of cloud-based software tools used for working with data, such as managing data in a data warehouse or analyzing data with business intelligence. Real-World Data can provide the most clear and comprehensive picture of significant areas of interest within the Life Sciences and Pharma industries, such as progression of diseases, responses of different groups of patients to treatments, and reimbursement patterns. Targeted RWE insights can be sold to providers, payers, researchers and other stakeholders as valuable information that supports scientific, medical and business decision-making. These insights can also, for example, serve as an important step in advancing personalized medicine by matching patients with treatments tailored to their individual needs.
Organizations are constantly thinking about the best ways to design customized portals to deliver both comprehensive and relevant information to their partners that can be used to drive innovation and development. The derivation of RWE insights from RWD presents a world of possibilities as we look towards the future of data monetization within the realm of Healthcare and Life Sciences. Real-World Data and Real-World Evidence are facilitating new opportunities with regards to clinical trial design, regulatory submissions and DaaS opportunities, and their full range of utility likely extends even further beyond the current scope of understanding.